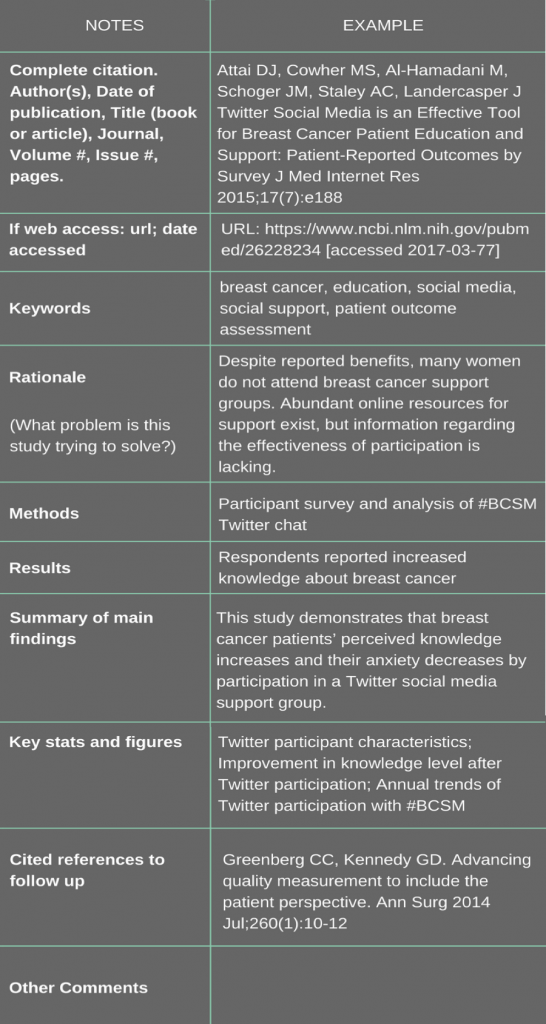
Overcoming Barriers to Accessing Small Cell Lung Cancer Care
Patient Empowerment Network (PEN) has a deep commitment to educate and empower patients and care partners in the lung cancer community. Lung cancer treatment options are ever-evolving with new testing and treatments, and it’s essential for patients and families to educate themselves with health literacy tools and resources on updated information in lung cancer care. With this goal in mind, PEN created the [ACT]IVATED Small Cell Lung Cancer program, which aims to inform, empower, and engage patients to stay abreast of lung cancer care updates.
The [ACT]IVATED Small Cell Lung Cancer program is geared to newly diagnosed lung cancer patients, yet it is beneficial for limited stage and extensive stage patients alike and for patient advocates. [ACT]IVATED Small Cell Lung Cancer helps patients and care partners stay abreast of the latest options for their lung cancer, provides patient activation tools to help overcome barriers to accessing care and powerful tips for self-advocacy, coping, and living well with cancer.
![SCLC [ACT]IVATED](https://powerfulpatients.org/wp-content/uploads/Screenshot-2024-01-09-at-1.22.18-PM-1030x584.png)
Small Cell Lung Cancer and Proactive Patients
Unfortunately, the stigma of lung cancer follows small cell lung cancer (SCLC) patients as well. Patient navigator Diana explained some of the history of lung cancer stigma. “Even though smoking is a major risk factor for SCLC, nobody deserves to get cancer. Nicotine is an addictive substance that is extremely difficult for many smokers to quit – especially for those who started at a very young age. Past TV ads to stop smoking built a stigma around cigarette smoking that has created an environment of blame around lung cancer. The stigma is many times greater for extensive stage small cell lung cancer patients.”
Advancing on the path to informed and optimal care requires patients to make efforts in self-education and empowerment. These efforts come in various forms but include approaches like improving clinical trial access, learning more from credible resources, asking questions to ensure your best care, and helping to educate others about lung cancer. Cancer patient Lisa Hatfield spoke with lung cancer expert Dr. Rafael Santana-Davila, Dr. Vinicius Ernani, and Beth Sandy to learn some key questions and actions for patients to take.
Small cell lung cancer falls under one of two categories – limited stage or extensive stage. Dr. Rafael Santana-Davila explained the distinguishing factors and the importance of communication between the medical team members. “In the majority of cases, there’s a very clear distinction, for example, patient has metastatic disease to the liver, that clearly is extensive, stage, but there are occasions where, limited and extensive is very hard to know…all of medicine is a team sport, but treatment of cancer is more because the medical oncologists need to talk to the radiation oncologists to make sure that we’re on the same page as to what is the best treatment we can offer a patient.“
It’s essential for SCLC patients and care partners to prepare themselves for the treatment journey to help ensure their best care. Dr. Santana-Davila shared some key questions to ask to empower themselves for treatment. “…key questions that families should ask at the outset of care, and this is for extensive stage cancer as well as any other cancer, is ‘What are the goals of treatment? What do I expect it to be? How is my life going to look a few months from now? And what can I expect?’ That is, for me, very important that patients know before they start on the journey of treatment.”
Thoracic medical oncology nurse practitioner Beth Sandy from Abramson Cancer Center shared patient advice for questions to ask at the outset of care to help patients empower themselves. “…make sure you know your stage, make sure you’re understanding what your treatments will be, and then make sure you understand what support services are available to you.”
Patients from underrepresented communities and all patients should ask questions to help ensure optimal care. Dr. Santana-Davila shared advice on proactive questions to ask. “’What are the latest developments in the treatment of this lung cancer? And am I eligible to receive those treatments? And is this a time where I should seek a second opinion or be referred to a clinical trial and another center?’”

Nancy Gatschet
Small cell lung cancer patients must be heard by their doctors for their best care. SCLC survivor and PEN Board Member Nancy Gatschet shared her experience with her care team members and their roles in her care. “Doctors matter. A lot. I was treated at an NCI-designated Comprehensive Cancer Center by several exceptional doctors. What made them exceptional? Their listening and observational skills first and foremost, their dedication to staying current with research, and their caring.”
Small Cell Lung Cancer Clinical Trials and Future Treatments
Clinical trials are vital for refining and advancing treatments for small cell lung cancer. Dr. Santana-Davila shared his perspective about clinical trials and also explained that many clinical trials can assist patients with transportation and lodging costs. “So it’s important for patients to consider clinical trials. That is where we’re analyzing the future medications, and many of those future medications will become the standard of care and by participating in clinical trials, patients will have access to those medications.”
Even though non-small cell lung cancer has had more treatment advancements in comparison to small cell lung cancer, that doesn’t mean that the future is bleak. Dr. Santana-Davila shared his perspective about the future of SCLC care and clinical trial opportunities. “So although it’s true that patients with non-small cell lung cancer have had more advances, there is still a lot of hope for the future. And what I can tell you it’s changing rapidly. And in a year, the treatments that we may have available will be different. And all those things are right now going into clinical trials.”
Dr. Vinicius Ernani from the Mayo Clinic sees a bright future for SCLC treatment as well. He shared his perspective with Lisa Hatfield, “…we have some important drugs coming in early development, like I mentioned before, ADCs, antibody drug conjugates. So my hope, that is we are going to be in a better spot in the near future.”
![SCLC [ACT]IVATED](https://powerfulpatients.org/wp-content/uploads/Screenshot-2024-01-09-at-1.25.27-PM.png)
[ACT]IVATED Small Cell Lung Cancer Program Resources
The [ACT]IVATED Small Cell Lung Cancer program series takes a three-part approach to inform, empower, and engage both the overall lung cancer community and patient groups who experience health disparities. The series includes the following resources:
[ACT]IVATED Animated Video Series
- Battling Small Cell Lung Cancer | One Man’s Journey
- Lessons From a Small Cell Lung Cancer Care Partner
- Small Cell Lung Cancer Care | Communication As a Key
- Moving Past Small Cell Lung Cancer Stigma | A Patient Navigator Explains
[ACT]IVATED Expert Interviews
- What Is the Difference Between Limited Stage and Extensive Stage Small Cell Lung Cancer?
- Key Questions to Ask About Extensive Stage Small Cell Lung Cancer
- What Treatment Options Are Available for Small Cell Lung Cancer?
- Small Cell Lung Cancer Care and Outcomes in Underrepresented Communities
- Small Cell Lung Cancer and Immuno-Oncology | What Patients Need to Know
- How Small Cell Lung Cancer Patients Can Best Self-Advocate
- Small Cell Lung Cancer | Hope for Treatment Advancements
- Key Resources for Small Cell Lung Cancer Patients and Families
- Advice for Small Cell Lung Cancer Patients Considering Clinical Trials
[ACT]IVATED Toolkit
- [ACT]IVATED SCLC Shared Decision Making Checklist
- SCLC Specialist Treatment Centers
- Expert SCLC [ACT]IVATION Tips
[ACT]IVATED Guides
Though there are small cell lung cancer challenges and stigma, patients and care partners can take action to educate themselves to help ensure optimal care. We hope you can benefit from these valuable resources to aid in your lung cancer care for yourself or for your loved one.