Myeloma Expert Explains Diagnosis and Treatment for Newly Diagnosed Patients from Patient Empowerment Network on Vimeo.
How can newly diagnosed multiple myeloma patients be oriented to their diagnosis and treatment? Dr. Sikander Ailawadhi from the Mayo Clinic shares key points he explains to patients about myeloma origination, tests, symptoms, treatment, and ongoing care.
Download Guide
Descargar Guía
See More from START HERE Myeloma
Related Programs:
Transcript:
Lisa Hatfield:
So now we’re going to jump into our questions. So, thank you again, Dr. Ailawadhi. So we have a patient asking for newly diagnosed patients, say a patient comes into you, maybe they were sent by their community oncologist or a family practitioner, something…I have myeloma, doesn’t know anything about it. Have even heard of it before. How do you start that conversation? How did you explain myeloma and the treatment and very importantly to the patient, how do you explain the prognosis when you know it’s not curable yet?
Dr. Sikander Ailawadhi:
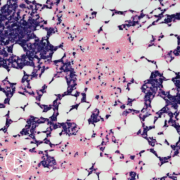
An extremely important question. And I agree that we should be starting at the beginning, so I think I had the privilege of working at an institution where we tend to spend a lot of face time with the patient, so typically in the outpatient, I have at least about an hour of time blocked is how we’re set up. So at that visit, first of all, I’m hoping that a patient comes in with a caregiver, but if they don’t have a caregiver with them, I start off by asking them, Is there someone they would like us to call during the visit? Because it is always better to have a caregiver or an extra set of ears listening in, and once that has started, then I typically will explain to them literally from what is a plasma cell, what is the role of a normal plasma cell, because that tells us the type of proteins plasma cells produce.
And that leads us to how a plasma cell can become cancerous and lead to multiple myeloma, what are the signs and symptoms of multiple myeloma? What are the markers, these protein markers that come in the blood and are picked up as markers of disease for patients, because again, patients need to know what they’re looking for in the labs that are drawn, so very frequently.
We talked about the role of a bone marrow biopsy, a lot of times it has been done, sometimes it has to be done after that visit, we talk about the genetic mutations in plasma cells that can be seen because that is what helps determine the risk category of standard risk or high risk.
I do offer to patients about discussing the prognosis, again, it’s a good time where we know that the average survival of patients is close to about 8 to 10 years when they look at a general national data, U.S. data, but all the large centers, all of us who focus on myeloma, we have several patients who are living quite a bit in excess of 10 years, so more hopeful time, but it is important to put that prognosis in perspective with high risk or standard risk disease that can be determined based on mutation testing from the plasma cells from the bone marrow, something called the FISH test, part of it is to explain to the patient the prognosis, but other reason is also because sometimes that can determine the type of treatment, and this also importantly tells the patients about their disease much better, so they can be more educated, they can interact with other patients, they can ask the right kind of questions, and they can understand their disease process and follow-up better.
Now, after we have discussed all of this, we start talking about treatment, I can tell you when I talk to a newly diagnosed patient, I will tell them that in my way of thinking their treatment initially is broadly divided into three different discussions during three different visits. The initial visit is talking about any symptom or sign from the myeloma, increased calcium, kidney dysfunction and tumors, how are we going to tackle that? So we will come up with the right “induction regimen.” I really don’t think one-size-fits-all, so based on the patient’s age, comorbidities, other diagnosis or the treatment drugs, family support system, financial situation, there are so many factors that go into it.
We come up with an induction regimen, I’ll tell them that the second component is about controlling all the symptoms and manifestations of the disease, whether that means radiation therapy, bone-strengthening agents, multivitamins, minerals, whatever we need to do as supplements, then we’ll talk about…starting that treatment. What does it involve? Side effects, we will set that path, you will notice I have not even talked about transplant, and I’ll tell the patients that only thing I mentioned to patients in that first planning, visitors and down the road, we will be talking about transplant. Today is not the time, because in my experience at the moment, we start talking about bone matter, transplant tenants, everything was out the window. That’s what patients think about…and I don’t want them to do that.
The second part of my discussion comes around a month or so into the treatment, because by then we want to start seeing some responses, some symptoms turning around, but that month two to three is very importantly the time to rebuild things. Does the patient need to go to physical therapy, pain control? Supportive or palliative care services? Lipoblasty or tuboplasty to strengthen their spine. I mentioned physical therapy, I’ll say it again, because I really think that’s very, very, very important for controlling the pain and supporting the movement and quality of life, managing any side effects, making sure that the dose is correct, do we need to tweak the doses, etcetera. And at that visit is tell them that, “Okay, very soon we will be talking about…we’ll be going into the details of a transplant, we will be passing along more information to you. But at your next visit, which would be probably at that two- to three-month mark, two- to three-cycle mark,” is when I will really sit and talk to them about our transplant…
So for me, the main transplant discussion comes on that cycle to recycle the two to three seconds have already got in patients feeling better, they are much more receptive for the next phase of treatment, which is when we talk about transplant, that’s how I do it, typically. And then we’ll explain a lot about what this transplant need…what does it involve? Caregiver needs a supportive care, vital organ testing, bone marrow biopsy, response depth, MRD, all of that.
So for me, this is kind of the journey that a patient, newly diagnosed patient goes through for the first few months, then their transplant, then their maintenance and hopefully good long disease control state.
Lisa Hatfield:
Great, how often do you expect a patient will have to have appointments during that…talk about the induction phase, the first month to three months, how often do you think they will have appointments, whether it’s for treatment or to come see you? What should they expect that way?
Dr. Sikander Ailawadhi:
Sure, so the regimens that we typically use in myeloma, some of them, the drugs are given twice a week, a majority of the way we give the drugs, it’s once a week, so one to two times a week would be visits, we do the labs for the first month, we will do sometimes every week, but by the time the patient has gone to the second or third cycle, once every two to four weeks, labs are reasonable because by then things have stabilized, but the treatment still would, I think the once or twice every week depending upon the regimen that they have, we don’t typically see the patient for a clinic appointment every time, but a lot of centers do, so every time the patient comes, as I said, one to two times a week, typically that translates to about four visits in every three to four weeks they coming on the cycle, some regiments are three weeks regiments, some regiments are four week regiments, etcetera.
So patients come, I can say that the first one to two months are most intensive for follow-up for labs, we wanna make sure everything’s been fine, been start reading the treatment, they are not having side effects it and etcetera, and then things can be spaced out a little bit for the next couple of months before we go into the transplant thing, if the patient is going for transplant.