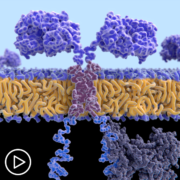
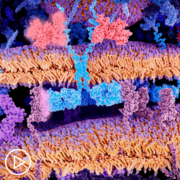
Thrive | Advice for Managing Potential CAR T-Cell Therapy Side Effects from Patient Empowerment Network on Vimeo.
Dr. Adriana Rossi, a myeloma expert and researcher, discusses how CAR T-cell therapy has revolutionized care, the process for undergoing this therapy, common side effects of this treatment, and advice for patients considering this option. Dr. Rossi also shares updates in CAR T-cell therapy research and explains what she’s excited about in myeloma care.
Dr. Adriana Rossi is Co-director of the CAR T and stem cell transplant program at the Center for Excellence for Multiple Myeloma at Mount Sinai Health System in New York City. Learn more about Dr. Rossi.
Download Resource Guide
See More From Thrive CAR T-Cell Therapy
Related Resources:
Transcript:
Katherine Banwell:
Hello, and welcome. I am your host, Katherine Banwell. Today’s program is part of our Thrive series, where we will discuss what to expect and how to manage side effects of CAR T-cell therapy.
Before we get into the discussion, please remember that this program is not a substitute for seeking medical advice. Please refer to your healthcare team about what might be best for you.
Well, let’s meet our guest today. Joining us is Dr. Adriana Rossi. Dr. Rossi, welcome. Would you please introduce yourself?
Dr. Adriana Rossi:
Thank you so much. I am one of the codirectors of the CAR T program at Mount Sinai in New York City and thrilled to be with you today.
Katherine Banwell:
Thank you. Since we’ll be discussing the ins and outs of CAR T-cell therapy, I thought we could start with your perspective as a researcher in the field. How has this therapy revolutionized myeloma care?
Dr. Adriana Rossi:
It absolutely has. And I would say in time we’ve had – this is now our fourth revolution. Stem cell transplants was the first time we actually achieved what we call a complete remission in at least a few patients, making myeloma disappear.
Then, we had the second revolution with the novel agents. Now, we had drug therapies that were giving us these complete remission still at about a 30 percent rate. And then, the monoclonal antibodies were the most recent revolution. And currently, we are in what we call the T-cell redirection.
It really has been driven by CAR T-cell therapies and something we call bispecific antibodies, which also use your patient’s T cells to kill the myeloma. We are now seeing absolutely unprecedented response rates, meaning almost everybody is responding. Also, depth of response, which we have really learned over time is a way to translate into long remissions. So, every long, very significant remissions. And the early data in patients who have had many prior lines.
Katherine Banwell:
So, it is very encouraging news.
Dr. Adriana Rossi:
It is very encouraging.
Katherine Banwell:
Let’s start with an overview of CAR T-cell therapy. Could you explain how the treatment works?
Dr. Adriana Rossi:
Absolutely. So, CAR T specifically is speaking to T cells, which are a normal part of the immune system that have been engineered and modified. So, normal part of the immune system T cells have a lot of checks and balances and are constantly looking for cells that are supposed to be killed. For example, something that has a virus in it.
When we engineer the CAR T-cells, we modify, one, the target so they are now trained to find the specific target on a tumor cell. And we remove all these checks and balances. So, once that T-cell finds its target, it can kill it without all of the side effects. The way normal T cells communicate with other members of the immune system are something called cytokines. So, we will touch on that a little later, I think, but we also, again, interfere with that communication by engineering the cells.
Katherine Banwell:
Which patient type qualifies for CAR T?
Dr. Adriana Rossi:
In 2023, we currently have two approved commercial CAR T products and we do have a number of them in clinical trials. The two that are commercially approved specifically are targeted for patients who are in their fourth line of therapy, so the myeloma has learned to come back that four times.
They’ve been exposed to all of the regular drugs, which by four lines most patients will have been at least once. We look for patients whose kidney function is at a safe level to tolerate the therapy. And other than that, it’s really having caregiver support and overall ability to come to a center that specializes in this.
Katherine Banwell:
What’s the process for accessing the CAR T?
Dr. Adriana Rossi:
The first important part is remembering they exist and having the referring physician remember to send patients our way. Once patients come to our center, they will meet with coordinators, both the clinic coordinators to make sure we have all of the testing, to make sure the heart is healthy enough, the lungs are healthy enough. There’s no infections brewing.
Financial coordinators to take care of all of the organizing. If patients are coming from further than 30 minutes, setting them up for a place to stay in the city, transportation aid, all of those things. Once we decide to go ahead and have our collection date set, that sort of starts the actual process. Since most of our patients have had stem cell transplants before, there is that point of comparison. I think one of the most important things to remember is CAR T is not stem cells.
So, while they’re both the cellular therapies, the patient experience is vastly, vastly different. It starts with a collection, where in stem cells you need several days of injections and maybe several days of collection. T-cell collection is a one-day event. We get what we get and then we are going to manufacture them and we can grow them in a Petri dish. There is no minimum and there is no instigating injections to get them going.
Once they’re collected, the cells are then sent for manufacturing, which may take from four to eight weeks. During that time, patients usually receive what we call a bridging therapy, which is some kind of therapy to keep the myeloma at bay. Not to get rid of it but to keep it under control so that once the cells are ready the patient is also ready. Going into CAR T with growing myeloma can increase the side effects.
Katherine Banwell:
Go ahead.
Dr. Adriana Rossi:
I will give you just the final bit. Once the cells are ready, then we plan to give chemotherapy to get the patient’s T cells to not put up a fight. That’s called lymphodepletion. We infuse the cells and they’re now with us for two weeks in the hospital and usually two weeks after.
Katherine Banwell:
Okay. So, I was going to ask how long patients are in the hospital for the procedure. So, that explains that. So, it is about two weeks. What signifies that a patient is ready to be released and go home?
Dr. Adriana Rossi:
The reason patients are in the hospital is a very classic expected toxicity experience. So, they’re in the hospital for us to observe, watch. If it happens, which about 80 percent of the time there will be some toxicity for us to address – one that toxicity has resolved, they’re then okay to go home.
Katherine Banwell:
Okay. That is great advice. Thank you. Of course, we know that CAR T-cell therapy comes with some potential side effects. Let’s talk about some of those side effects and how they’re managed. You mentioned cytokine release syndrome earlier. Let’s start with that. What is it, exactly?
Dr. Adriana Rossi:
Yes. As I mentioned, cytokines are molecules that the cells of the immune system use to communicate with each other. With this therapy, we are asking the T cells that have been infused to expand, meaning make multiple copies of themselves, and sweep through the body looking for myeloma and basically picking a fight with them.
So, CRS is what happens when the T cells are too good at their job and they overachieve and then picking a little fight kind of make a big ruckus. The result is what we call inflammation, which the patient will experience usually as a fever.
But if it does not go – if it continues to go unchecked, that fever can be accompanied by low blood pressure because of these inflammatory markers, difficulty breathing or low oxygen levels. And all of these things are now vastly prevented. CRS is usually treated very quickly and doesn’t get to these higher grades, more complicated fields.
Katherine Banwell:
How is CRS managed?
Dr. Adriana Rossi:
We have a couple of very good antidotes. CRS by itself is not just a fever. Certainly, a fever in any patient who is undergoing these kinds of therapies, we will try to rule out any infections. But there are markers in the blood that we can follow. When the blood markers and the fever occur at the same time, we know that cytokines are driving that effect. If it seems to be driven by something we call IL-6, we use tocilizumab (Actemra). If it seems to be driven by IL-1, we use anakinra (Kineret). These are all drugs that are themselves monoclonal antibodies which then will shut down that overreaction and cool things down.
Katherine Banwell:
Okay. Another possible side effect is neurotoxicity. Would you define that term for us?
Dr. Adriana Rossi:
Yes. That one is harder to define because neurotoxicity in itself is very broad. We usually think of something called ICANS, which is the neurotoxicity associated with the effector cells. That specific neurotoxicity tends to happen in conjunction with CRS.
And while CRS probably occurs in about 85 percent of patients, the ICANS is usually in the order of 5 percent. So, much, much more rare. And the antidote for that, which most patients know, love, and hate, is steroids.
Katherine Banwell:
Ah, yes.
Dr. Adriana Rossi:
I should mention there are other parts of neurotoxicity which I think the most concerning is something that has been known as Parkinsonian symptoms. It’s really just movement disorder. These are exceedingly rare and so we haven’t had a chance to learn very much because there are so few patients who have had this complication. We have learned from the first six patients who had this how to avoid it. And so, I think it’s now even more rare and it really goes into patient selection, to making sure, as I mentioned, that the myeloma isn’t growing very much.
We monitor to see if the T cells grow too fast, if the CRS is of a high level. These are all predictors of delayed neurotoxicity.
Katherine Banwell:
What are the signs of neurotoxicity in a patient?
Dr. Adriana Rossi:
Very specifically, for the ICANS, we have tool called the ICE tool, which is a series of questions to test memory and attention and ability to write and understand and speak. So, most commonly, it would be an inability to speak properly or, if someone is writing a sentence, it’s really a very classic finding. It is no longer spread across the page.
These are not subtle findings. Part of, again, being in the hospital is to allow us to have this tool twice a day and look for these signs very early on, interfere with their development by giving the patients steroids – usually for a day or two – and resolving it.
Katherine Banwell:
So, that’s how neurotoxicity is managed, then.
Dr. Adriana Rossi:
Yes.
Katherine Banwell:
And is there a potential for long-term issues associated with neurotoxicity?
Dr. Adriana Rossi:
Certainly, there is always the potential. But the vast majority – again, the ICANS tend to be self-limited while the patient’s in the hospital, and that is why we’re watching during that window. The delayed neurotoxicities, in addition to these very rare movement disorders, we do see some cranial nerve palsies. The seventh cranial nerve, usually recognized as Bell’s palsy, has happened a few times. We really don’t understand the mechanism of what is driving it. It’s inflammation but why there, why that way. So, we tend to use acyclovir, which is the classic treatment for Bell’s palsy and steroids.
Katherine Banwell:
Dr. Rossi, a suppressed immune system is something that a patient undergoing CAR T-cell therapy should consider. What does it mean and what precautions should patients take?
Dr. Adriana Rossi:
That is such a good question and it is specifically true for patients who are receiving therapies that target BCMA, which both commercial CAR Ts at the moment target.
Because it is such an effective therapy at bringing down cells that express BCMA, your immune cells that make antibodies, one of the side effects is the immunoglobulins, which are the antibodies, are all very, very low. So, that is one level of immunosuppression.
The other is the chemotherapy that we use to quiet the T cells can also lower all the blood counts. So, red blood cells and platelets may be low as well and those are not involved with immunity and can be transfused. So, that is a supportive mechanism. For the immune therapies, we usually use IVIG, which is intravenous immunoglobulins to support the patient until they’re able to make their own.
We also protect them from viral infections with acyclovir or valacyclovir. Protect them from something called PJP pneumonia, which is a virus that specifically appears when you’re very immunosuppressed. Should their neutrophil count be low, that is another type of white blood cell – make sure they’re protected with antibiotics.
Katherine Banwell:
Is there a typical timeframe for the immune system function to return?
Dr. Adriana Rossi:
I would say a year is a good time but it’s a very unpredictable wave. So again, unlike stem cell transplant where you have a clear time where the cells are low, they recover, they stay recovered, we have noticed for some patients, they may have low blood counts just during the first month and then be recovered. Some will have no problems in the first month and it’s in the weeks to follow that suddenly either the reds, or the platelets, or the white count may need support.
And in very rare instances, out to a year, they’re still needing support, sometimes say a growth factor injection once a week.
Katherine Banwell:
So, how is it monitored over time?
Dr. Adriana Rossi:
We monitor all those different levels of the immune system. So, we check on the CBC, which is the very common blood counts. We also look at what is called a lymph panel to look at the different types of T cells and make sure that they are recovering. Those usually take about three to six months to recover. The white count, again usually by Day 30, but there are some cases of delayed recovery. And the immunoglobulins, which is the antibody level, we also monitor monthly.
Katherine Banwell:
What other side effects should patients who are considering CAR T-cell therapy be aware of?
Dr. Adriana Rossi:
Really, those are the big three. I would say others are very rare but the low blood counts is the one that lasts beyond the time in the hospital. And the rare neurotoxicities that are delayed.
Katherine Banwell:
When should patients mention any issues they’re experiencing to their healthcare team?
Dr. Adriana Rossi:
Always. That is a very, very, short answer. Please don’t ever think you are bothering the doctor. I hear that a lot. “Oh, I didn’t want to bother you.” It is never a bother. This is why we are here. So, anything that is happening that is out of the ordinary, please let your healthcare provider know. If it is not something that needs our attention or we don’t need to worry about, we will tell you.
Katherine Banwell:
Better safe than sorry.
Dr. Adriana Rossi:
Always.
Katherine Banwell:
And how does a care partner factor into the process? It seems having a good support system is essential.
Dr. Adriana Rossi:
It absolutely is. I think the entire journey of myeloma really is what I would consider a team sport. It is not something we go through alone. And the more members of the team you have the better. So, as your medical team, we always value the caregivers. For CAR T specifically, since there is this concern for infections and neurotoxicity, caregivers are really essential. They should be well informed, know what to look for, and be the ones to reach out to us if anything is concerning. Again, any symptoms out of the ordinary, any fever, and really be a part of communicating with the medical team.
Katherine Banwell:
Is there a period where patients are considered out of the woods from CAR T side effects?
Dr. Adriana Rossi:
Hard to say. Again, I like to emphasize that most patients by Day 30 or 60 are back to work, are feeling themselves, are recovered. Another contrast to stem cell transplants. It’s a much faster recovery. I have patients who within 30 days are eager to go back to work and don’t know what I was talking about or why I insist on seeing them so much.
But some patients, again, out to a year, may still be requiring visits for support in either the IVIG for the immunoglobins, growth factor support for their counts. So, there are outliers at both extremes. We follow the model of 100 days for recovery.
Katherine Banwell:
Do some patient types do better than others?
Dr. Adriana Rossi:
Well, always yes. And we are still endeavoring to figure out who they are and why that is. There are things that we don’t know, can’t predict. But things that we do recognize are again bringing patients whose myeloma is under good control.
So, instead of having a lot of disease or disease that is in a growth phase, we try to use the bridging therapy to optimize the patient, not only to improve the response, but also minimize the toxicities.
Katherine Banwell:
Does age have an impact at all?
Dr. Adriana Rossi:
Not as much. We actually have just finished an 88-year-old patient whose hospital course was remarkably unremarkable, as we would like. I think another difference from stem cells, it is not as rigorous. While each patient, I think, should be part of that decision and that conversation, reviewing what is now a growing number of options and see if it’s right for them as an individual. So, age is a consideration, but frailty will always be the more important.
Katherine Banwell:
Dr. Rossi, we discussed the process of accessing CAR T-cell therapy, which can be a big undertaking. How do you counsel patients who are considering this treatment option?
Dr. Adriana Rossi:
Mostly, I want to make sure that they are well-educated and understand as much as we do and as much as we can convey. I am fortunate to be part of a big multidisciplinary team so there is social workers, clinical coordinators, other specialists, dentists, cardiologists, to give all of the perspectives. I like to make sure that they know what it is and also that they know what it isn’t. So, it is not a stem cell transplant and it is not another line of therapy that you just sign up for and go into blindly.
So, making sure they’ve had all of their questions answered, and it’s not something they read on the Internet. They have spoken with one of the CAR T physicians, understand all of the steps of the process, and have questions to their very individual needs addressed.
Katherine Banwell:
If a patient is interested in possibly doing CAR T-cell therapy, what questions should they ask their healthcare team?
Dr. Adriana Rossi:
I think again making it personal to them. Does the team think they are a good candidate? Is this the right time? Because they may be a good candidate but not even need it at the moment. Or, again, there are things that we could do between now and the cells to optimize the success both in efficacy and toxicity.
Understanding what side effects are expected for that individual because, again, we can usually judge these will be more likely or less likely. And then, do I have a plan in place to find the right center and continue the care and the monitoring near home after that?
Katherine Banwell:
What are the alternatives if a patient decides CAR T is not right for them?
Dr. Adriana Rossi:
I would say as part of this newest revolution and fairly comparable in novelty and method of action would be the bispecific antibodies. So, these are molecules.
They are not cells. And they activate the patient’s own T cells and bring the T cells to the myeloma, causing very similar side effect profile and very similar effectiveness. The rates are a little bit lower but they are administered as mostly a subcutaneous injection that has to be dosed weekly or every other week. The contrast is it’s a continuous therapy, but it does allow us to adjust as we go, which the cellular therapy doesn’t.
Katherine Banwell:
While there are approved CAR T-cell therapies for myeloma currently, there are also many others that are in clinical trials. Would you talk about some of the ongoing research in this area?
Dr. Adriana Rossi:
Absolutely. Again, while we celebrate the tremendous changes that these two CAR Ts have made to the field, they are both autologous, meaning we use the patient’s own T cells for manufacturing. They both target BCMA. And they are both what we call second generation T cells. So, other areas are to change the target. So, instead of just targeting BCMA, there are studies specifically targeting GPRC5D, which are coming down fairly soon. Rather than using the patient’s own T cells there are a number of products that use a healthy donor’s T cells, which are available immediately.
So, we don’t need to go through the bridging therapy, and we don’t have to wait for the cells to be ready. And lastly, there are different manufacturing processes. As I mentioned, the ones we currently have may take up to eight weeks for manufacturing. There are some studies now where cells are basically manufactured, engineered, in 48 hours –
Katherine Banwell:
Oh, wow.
Dr. Adriana Rossi:
– and are ready to be infused so that they actually grow in the patient rather than in a Petri dish. So, lots of areas of exploration and I look forward to, in five years, being able to look back and see again how the field has changed.
Katherine Banwell:
And I’m sure it will, by the sounds of it. Are there any trials introducing CAR T-cell therapy as an earlier line of myeloma treatment?
Dr. Adriana Rossi:
There are. So, both the products that are now commercially available for the fourth line are being studied in earlier and earlier lines. We actually just this year got results of the CARTITUDE-4 study, which was in one to three prior lines, and expect that that will lead to an earlier approval in the very near future.
And we have a number of studies, again, with both products looking at patients who have either high risk disease or don’t respond as well as we would like to their frontline therapy, and actually being used as part of that first line.
Katherine Banwell:
Dr. Rossi, what advice do you have for patients who may be hesitant to participate in a clinical trial?
Dr. Adriana Rossi:
Education. More than anything, understand what they are. Clinical trials come in all shapes and sizes. We have these exciting molecules that have to go into a first human at some point but we also have tried and true therapies that we know – for example, the CAR T – that is approved in these later lines. That same product is being now offered earlier. So, that has to be within a clinical trial because it’s not the approved indication.
But it is a product that we know to be safe. We know that it works in advanced disease and are actually expecting that it will work even better in earlier lines. So, clinical trials is a very broad term. Understanding what the patient may be eligible for – meaning, what the study’s looking for – and then comparing that to what the patient is looking for. So, sometimes it’s even modes of therapy. So, if you’re specifically looking for an oral agent, there may be studies that don’t require injections or that many visits. So, really looking widely, speaking to your healthcare physician, and understanding what the options are.
Katherine Banwell:
And if a patient is interested in possibly participating in a clinical trial, what sorts of questions should they ask?
Dr. Adriana Rossi:
Very, very good question. First, understanding what clinical trial. Each center will have their own combination. Some studies are available in multiple locations. Some studies are very institution specific. So, meeting with the research team and understanding what are the required testings, what is the required treatments, and what is the required follow-up, I think, is the first part.
Clinical trials, in order for them to give us the power to generalize and learn lessons are very strict in trying to keep to the schedule just as specified and everything is much more contained. So, making sure that they again understand what they’re signing up for and what they’ll get out of it.
Katherine Banwell:
What other myeloma research are you excited about?
Dr. Adriana Rossi:
Well, my focus is in CAR T and so I think, with bias, that is the most exciting part. But I did mention bispecifics. One of the things we need to concede is CAR T really requires you be at a cellular therapy center.
Whereas, with the bispecifics, while for now experience is still building, the idea is that this is something that could be administered in any practice across the nation. So, being able to reach more patients and those also with different targets, different schedules, different combinations, was another very interesting field as well.
Katherine Banwell:
As we close out this conversation, Dr. Rossi, I would like to get your take on the future of myeloma. What makes you hopeful?
Dr. Adriana Rossi:
Just looking back, I think. Again, in the 20 years that I’ve been fortunate enough to participate and see the changes, we have gone through, as I mentioned, three of the four revolutions in the field. And the speed with which each step forward then begets three or four more. As I mentioned, in five years I think we’ll look back and say, “Oh, how quaint, what we were doing in 2023.” So, the speed and the number of wins we’re getting and how quickly that’s translating into direct patient experience is really incredible.
Katherine Banwell:
Yeah. It seems like there’s a lot of progress and hope in the field.
Dr. Adriana Rossi:
There absolutely is.
Katherine Banwell:
Well, Dr. Rossi, thank you so much for taking the time to join us today.
Dr. Adriana Rossi:
Absolutely. It’s been my pleasure.
Katherine Banwell:
And thank you to all of our collaborators. To learn more about myeloma and to access tools to help you become a proactive patient, visit powerfulpatients.org. I’m Katherine Banwell. Thanks for being with us today.